Complex Disordered Systems
Surfactants
Francesco Turci
Hydrophobicity and amhiphiles
Water is the standard solvent for a lot of chemistry and (almost all) biology.
Molecules in water can be classified by their affinity to water:
- Hydrophilic (“water-loving”) molecules interact favorably with water (e.g., via hydrogen bonding or dipole interactions).
- Hydrophobic (“water-fearing”) molecules do not interact favorably with water (e.g., non-polar molecules).
Amphiphiles are molecules that contain both hydrophilic and hydrophobic parts. They include:
- Surfactants (detergents, soaps, SURFace ACTive AgeNT)
- Lipids (cell membranes)
- Some proteins (with hydrophobic and hydrophilic amino acids)
Schematic model of a surfactant
Hydrophobicity and amphiphiles
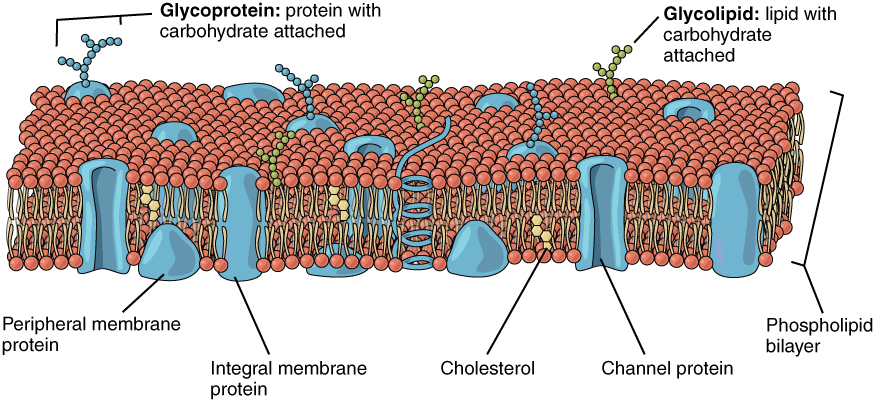
Phospholipid bilayers form the cellular membranes. They self-assemble!
Hydrophobicity in brief
The hydrophobic interactions is a topic of active research.
Various approaches
- (from to 1950s) structural approach: water forms cages around molecules. When the cage is disrupted, entropy increases. Solutes come together to minimise the disruption.
- (from 2000s) interfaces approach: water is a fluctuating medium. Large hydrophobic solutes create interfaces that suppress fluctuations. Solutes come together to minimise the interface area.
- (current research) avoided criticality: water is close to a liquid-vapour critical point. Local curvature and interactions promote large density fluctuations. Solutes interact on scales controlled by these fluctuations.
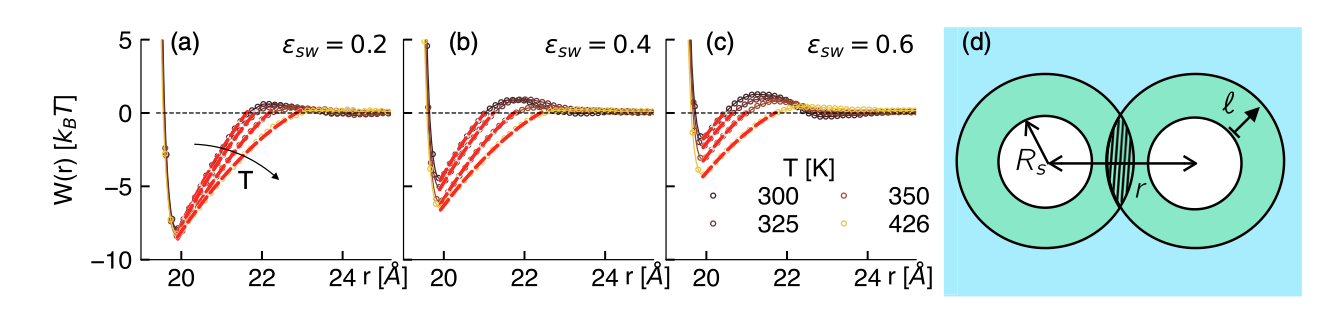
Effective hydrophobic interactions between spherical solutes of variable solute-solvent affinities \varepsilon_{sw}. A depletion-like construction produces a surface tension-controlled interaction. from Wilding & Turci PRR (2025)
In all cases, the hydrophobic effect originates in fluctuations of the solvent, i.e. the way the solvent explores the available configuration.
Self assembly of surfactants
Code
// This is ObservableJS code
// You can run at observableehq.com or convert it to an equivalent (and faster?) Python version if you like
viewof simulation = {
// --- Parameters ---
const width = 40, height = 40;
const chainLength = 3;
const chainsToCreate = numChains;
const T = temperature;
// const numChains = 10;
const E_TS = 1.0; // tail-solvent energy (solvophobic)
const E_HS = -1.5; // head-solvent energy (solvophilic)
// const T = 0.1; // temperature (kT units)
// --- Initialize grid and chains ---
let grid = Array.from({length: width}, () => Array(height).fill(null));
let chains = [];
// Periodic boundary helper
function mod(n, m) { return ((n % m) + m) % m; }
function getNeighbors(x, y) {
return [
{x: mod(x - 1, width), y: y},
{x: mod(x + 1, width), y: y},
{x: x, y: mod(y - 1, height)},
{x: x, y: mod(y + 1, height)}
];
}
function placeChains() {
for (let id = 0; id < numChains; id++) {
for (let tries = 0; tries < 100; tries++) {
let x = Math.floor(Math.random() * width);
let y = Math.floor(Math.random() * height);
if (grid[x][y]) continue;
let chain = [{x, y, type: 'head'}];
grid[x][y] = {id, type: 'head'};
let ok = true;
for (let i = 1; i < chainLength; i++) {
let last = chain[chain.length - 1];
let nbs = getNeighbors(last.x, last.y).filter(p => !grid[p.x][p.y]);
if (nbs.length === 0) { ok = false; break; }
let next = nbs[Math.floor(Math.random() * nbs.length)];
chain.push({...next, type: 'tail'});
grid[next.x][next.y] = {id, type: 'tail'};
}
if (ok) { chains.push({id, segments: chain}); break; }
else { chain.forEach(p => grid[p.x][p.y] = null); }
}
}
}
function energy(seg) {
let solventNbs = getNeighbors(seg.x, seg.y).filter(p => !grid[p.x][p.y]).length;
return seg.type === 'head' ? solventNbs * E_HS : solventNbs * E_TS;
}
function attemptMove(chain) {
const forward = Math.random() < 0.5;
const tail = forward ? chain.segments[0] : chain.segments.at(-1);
const head = forward ? chain.segments.at(-1) : chain.segments[0];
const options = getNeighbors(head.x, head.y).filter(p => !grid[p.x][p.y]);
if (options.length === 0) return;
const next = options[Math.floor(Math.random() * options.length)];
const dE_old = energy(tail);
grid[tail.x][tail.y] = null;
const dE_new = energy({...next, type: tail.type});
grid[tail.x][tail.y] = {id: chain.id, type: tail.type};
const deltaU = dE_new - dE_old;
const accept = deltaU < 0 || Math.random() < Math.exp(-deltaU / T);
if (accept) {
grid[tail.x][tail.y] = null;
grid[next.x][next.y] = {id: chain.id, type: tail.type};
if (forward) {
chain.segments.shift();
chain.segments.push({...next, type: tail.type});
} else {
chain.segments.pop();
chain.segments.unshift({...next, type: tail.type});
}
}
}
// --- Visualization ---
const svg = d3.create("svg")
.attr("viewBox", `0 0 ${width} ${height}`)
.style("width", "400px")
.style("height", "400px")
.style("border", "1px solid #ccc");
const g = svg.append("g");
function draw() {
const data = chains.flatMap(c => c.segments);
g.selectAll("circle")
.data(data, d => `${d.x}-${d.y}`)
.join("circle")
.attr("cx", d => d.x + 0.5)
.attr("cy", d => d.y + 0.5)
.attr("r", 0.45)
.attr("fill", d => d.type === "head" ? "blue" : "red");
}
// --- Main Loop ---
placeChains();
draw();
let running = true;
const button = html`<button>⏸ Pause</button>`;
button.onclick = () => {
running = !running;
button.textContent = running ? "⏸ Pause" : "▶ Resume";
};
(async () => {
while (true) {
if (running) {
for (let i = 0; i < chains.length; i++) {
const c = chains[Math.floor(Math.random() * chains.length)];
attemptMove(c);
}
draw();
}
await new Promise(r => setTimeout(r, 50));
}
})();
return html`<div>${button}<br>${svg.node()}</div>`;
}Self assembly of surfactants
Surfactants are at the molecular scale so they are nanometer sized
- Diffusion is fast (e.g. use D=\frac{k_B T}{6 \pi \eta R} to compare a colloid to a surfactant molecule)
- \to quickly explore possible conformations and minimise free energy.
The amphiphilic structure means that special orientations can be achieved to satisfy the energetic constraints.
The molecules spontaneously form structures: this is called self-assembly.
Underlying phase transitions typically drive the self-assembly.
Aggregation: general case
Consider a generic problem of aggregation of particles (generic)
Simple model: we have cluster of size N and a free energy cost \epsilon_N pto add a particle to the cluster.
Let \mu_1 and \mu_N be the chemical potential of isolated particles and aggregates of size N respectively.
In equilibrium, \mu_1 = \mu_N := \mu
We can express \mu in terms of
- the interaction energy from being in the aggregate \epsilon_N
- the entropy of the aggregate as a whole \propto {\text{ number of aggregates}}\times \ln {\text{ number of aggregates}}
Aggregation: general case
- Let the overall volume fraction to be \phi
- Call X_N the volume fraction in an aggregate of size N, so that \sum_N X_N = \phi.
- The numbers of aggregates of size N per unit volume is then simply X_N/N
Example
- Suppose \phi = 0.1 (10% of the volume is particles).
- Let X_3 = 0.06, X_2 = 0.04 (so \sum X_N = 0.06 + 0.04 = 0.1 = \phi).
- Then the number of trimers is 0.06 / 3 = 0.02 (fraction of solution volume in separate trimer aggregates).
- The number of dimers is 0.04 / 2 = 0.02.
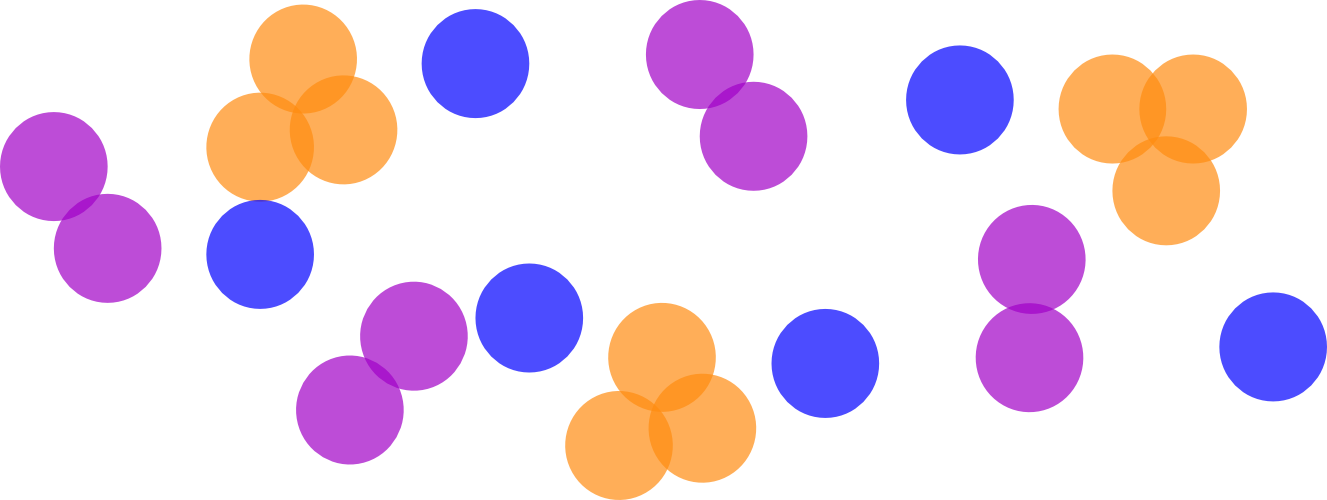
___
The uniform chemical potential is then
\mu = \text{\{energy per particle in aggregate of size N\}} + \text{\{entropy of mixing per particle in aggregate of size N\}}
or \mu = \epsilon_N + \dfrac{k_B T}{N}\ln{\dfrac{X_N}{N}}
Aggregation: general case
From \mu = \epsilon_N + \dfrac{k_B T}{N}\ln{\dfrac{X_N}{N}}
we can rewrite this as
X_N = N \exp{\left( \dfrac{N(\mu-\epsilon_N)}{k_BT}\right)}
and eliminate \mu by evaluating the expression for for N=1 and plugging it back to get
X_N = N X_1^N \exp{\left( \dfrac{N(\epsilon_1-\epsilon_N)}{k_BT}\right)}
The fraction of solutes in the aggregated state is large only if \epsilon_1>\epsilon_N., i.e. there is an energetic advantage.
Aggregation: general case
X_N = N X_1^N \exp{\left( \dfrac{N(\epsilon_1-\epsilon_N)}{k_BT}\right)}
means that knowing the form of \epsilon_N is key to predicting aggregation behaviour.
- Consider an aggregate of N particles of total radius r\approx (Nv)^{1/3} where v is the volume of a single particle.
- Then \epsilon_N is the free energy per particle of the aggregate of size N, G_N/N=\dfrac{1}{N}(\text{ bulk free energy}+\text{surface free energy})
- Assuming that there is a surface free energy cost (a surface tension) \gamma we write
\epsilon_N = \dfrac{G_N}{N} = \epsilon_\infty +\dfrac{1}{N}\gamma r^2 =\epsilon_\infty +\gamma\left(\dfrac{v^2}{N}\right)^{1/3}
which is a monotonically decreasing function of N.
Define \alpha k_B T = \gamma v^{2/3} and extract a relation between X_N and X_1 parametrised solely by \alpha, i.e.
\text{number of aggregates of size N per unit volume} = \dfrac{X_N}{N}\sim (X_1 e^{\alpha})^N
Aggregation: general case
\text{number of aggregates of size N per unit volume} = \dfrac{X_N}{N}\sim (X_1 e^{\alpha})^N This presents us with a few cases depending on \alpha= \gamma v^{2/3}/k_B T
- X_1 e^{\alpha} < 1: Few isolated particles, and (X_1 e^{\alpha})^N becomes vanishingly small for large N
- X_1 e^{\alpha} = 1 (critical point): Aggregates of all sizes become equally probable. The system undergoes phase separation into a dilute phase of isolated monomers (with X_1 pinned at e^{-\alpha}) in coexistence with a dense phase of very large aggregates.
- X_1 e^{\alpha} > 1: Above the critical aggregation concentration, the system cannot remain homogeneous and phase separates. The volume fraction \phi at which this occurs is called critical aggregation concentration, or CAC.
\text{CAC} = X_1^* = e^{-\alpha} = \exp\left(-\frac{\gamma v^{2/3}}{k_B T}\right)
Aggregation: general case
Aggregation: surfactants case
Earlier we assumed that the free energy per particle was \epsilon_N = \epsilon_\infty +\gamma\left(\dfrac{v^2}{N}\right)^{1/3} a monotonically decreasing function of N.
For surfactants, it is not necessarily the case.
- Typically \epsilon_N has a minimum at some finite N=N^* resulting from the fact that beyond a certain size, the aggregate becomes unfavourable (e.g., due to packing constraints, curvature energy, etc).
- Therefore the distribution of aggregates of size N is peaked around N^*.
- This leads to the formation of micelles: aggregates of a characteristic size.
Aggregation: surfactants case
Surfactants in solution: normally, this is done in water in the presence of an interface with air. Due to their nature as amphiphiles, the surfactants typically sit at the air-water interface in a dynamical, equilibrium process that exchanges monomers between the bulk and the surface. The bulk surfactants, when the concentration is larger than a critical value, also self-assemble into micellar structures, which are at equilibrium with the isolated single surfactants.
Critical micellar concentration (CMC)
The addition of surfactants to water initially reduces the water-air surface tension, but when the surface is saturated, further addition leads to crossing the a critical micellar concentration (CMC) and micelle formation.
- Below the CMC, almost all surfactant molecules are present as monomers
- Above the CMC, additional surfactant molecules predominantly form micelles of size N^* while the monomer concentration remains nearly constant.
The aggregation in water follows specific patterns:
- hydrophobic tails cluster together to avoid water
- hydrophilic heads face the water
The structure on the micelle surface (formed by the heads) is disordered and fluid-like, similar to a 2D liquid.
Schematic of micelles (from DataPhysics Instruments).
Critical micellar concentration (CMC)
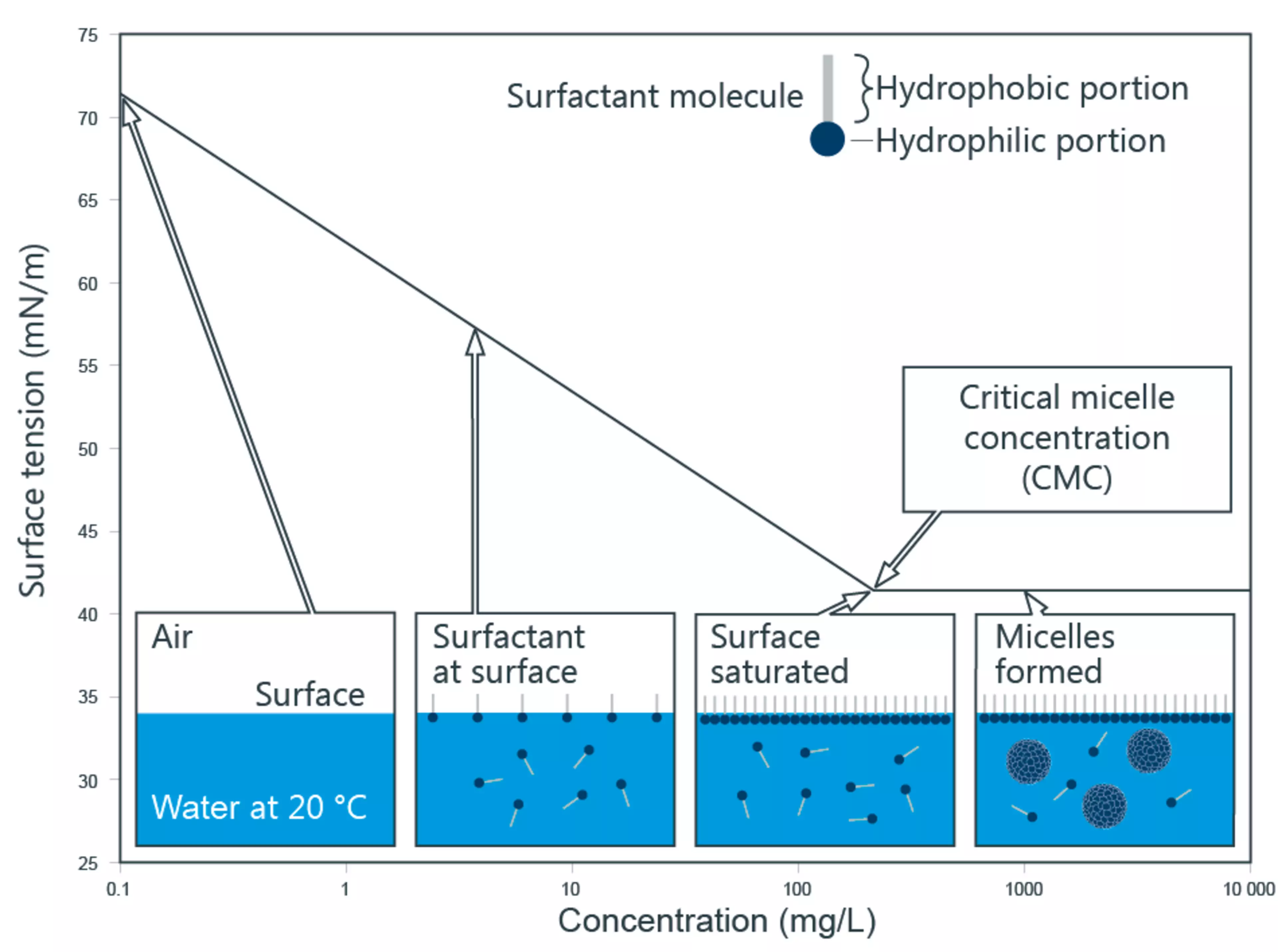
Surface tension of a surfactant solution with increasing concentration, formation of micelles, from Kruss Scientific
Shape of surfactant assemblies
Geometric model of a surfactant molecule:
- optimal headgroup area a_{0}: depends on chemical structure, pH and temeprature.
- volume v of the hydrophobic part: The hydrophobic part usually consists of hydrocarbon chains. Its volume v\propto n the number of carbon atoms in the chain.
- critical chain length \boldsymbol{l}_{\boldsymbol{c}} : the effective length of the chain, taking into account chain flexibility, temperature, branching and chemical structure. It also scales like l_c \propto n.
A balance between energy and entropy leads to different self-assembled structures .
Different types of self assembled structures: (A) spherical micelles, (B) cylindrical micelles and (C) bilayers.
Spherical micelle
For a spherical micelle of radius R with aggregation number N , the total volume and surface area are given by
\begin{gathered} N v=\frac{4 \pi}{3} R^{3} \\ N a_{0}=4 \pi R^{2}\end{gathered}
Therefore \dfrac{v}{a_{0}}=\dfrac{R}{3}<\dfrac{l_{c}}{3}
where we used the fact that the radius R cannot be larger than the critical chain length \mathrm{l}_{\mathrm{c}}. We thus obtain for the critical packing parameter P
P=\frac{v}{a_{0} l_{c}}<\frac{1}{3}
Cylindrical micelles
For a cylindrical micelle of length L the total volume and surface area are given by N v=\pi R^{2} L
and
N a_{0}=2 \pi R L
Therefore \dfrac{v}{a_{0}}=\dfrac{R}{2}<\dfrac{l_{c}}{2} again using R<l_{C}.
We thus obtain for the critical packing parameter P=\dfrac{v}{a_{0} l_{c}} that \dfrac{1}{3}<P<\dfrac{1}{2}
Below the lower limit spherical micelles are formed.
Bilayers (lamellar)
For a bilayer (with separation D between the layers and area A) the total volume and surface area are given by
\begin{aligned} & N v=A D \\ & N a_{0}=2 A \\ & \frac{v}{a_{0}}=\frac{D}{2}<l_{c} \quad\left(\text { using } D<2 l_{c}\right) \end{aligned}
We thus obtain for the critical packing parameter P=\dfrac{v}{a_{0} l_{c}} that \dfrac{1}{2}<P<1
Below the lower limit cylindrical micelles are formed.
Packing parameter summary
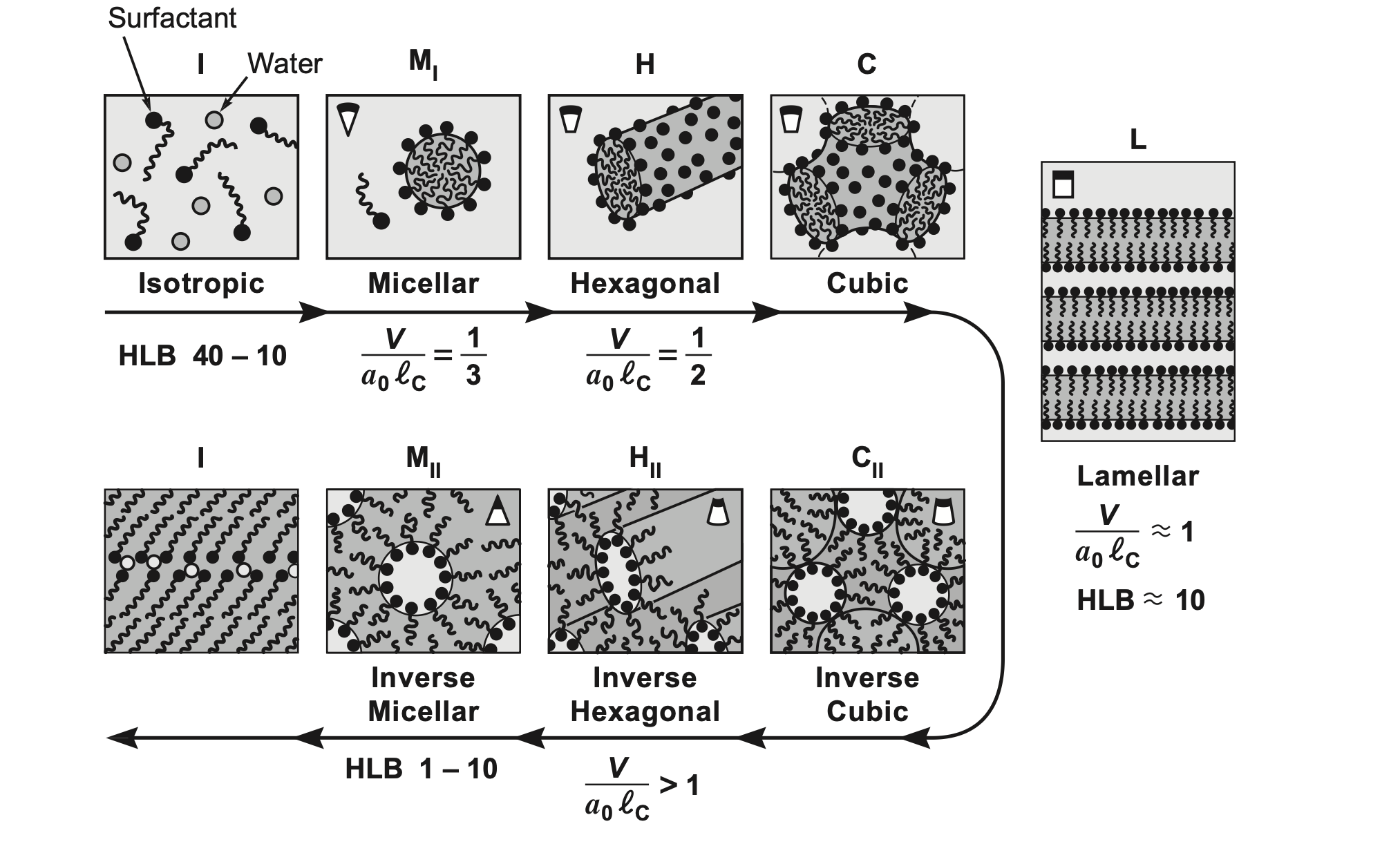
Mesophases formed by surfactants as we vary the packing parameter P=\dfrac{v}{a_{0} l_{c}} , from Israelachvili, J. N., Intermolecular and Surface Forces, 3rd Edition, Academic Press, 2011.